abbott rapid covid test
Panbio COVID-19 Ag Rapid Test Device Code. Panbio COVID-19 Ag Rapid Test Device Nasopharyngeal with 2D barcode 25T 41FK20.

Abbott Labs Rolls Out Rapid Covid Test To Us Schools And Workplaces
Abbott is bringing BinaxNOW the most affordable and widely.
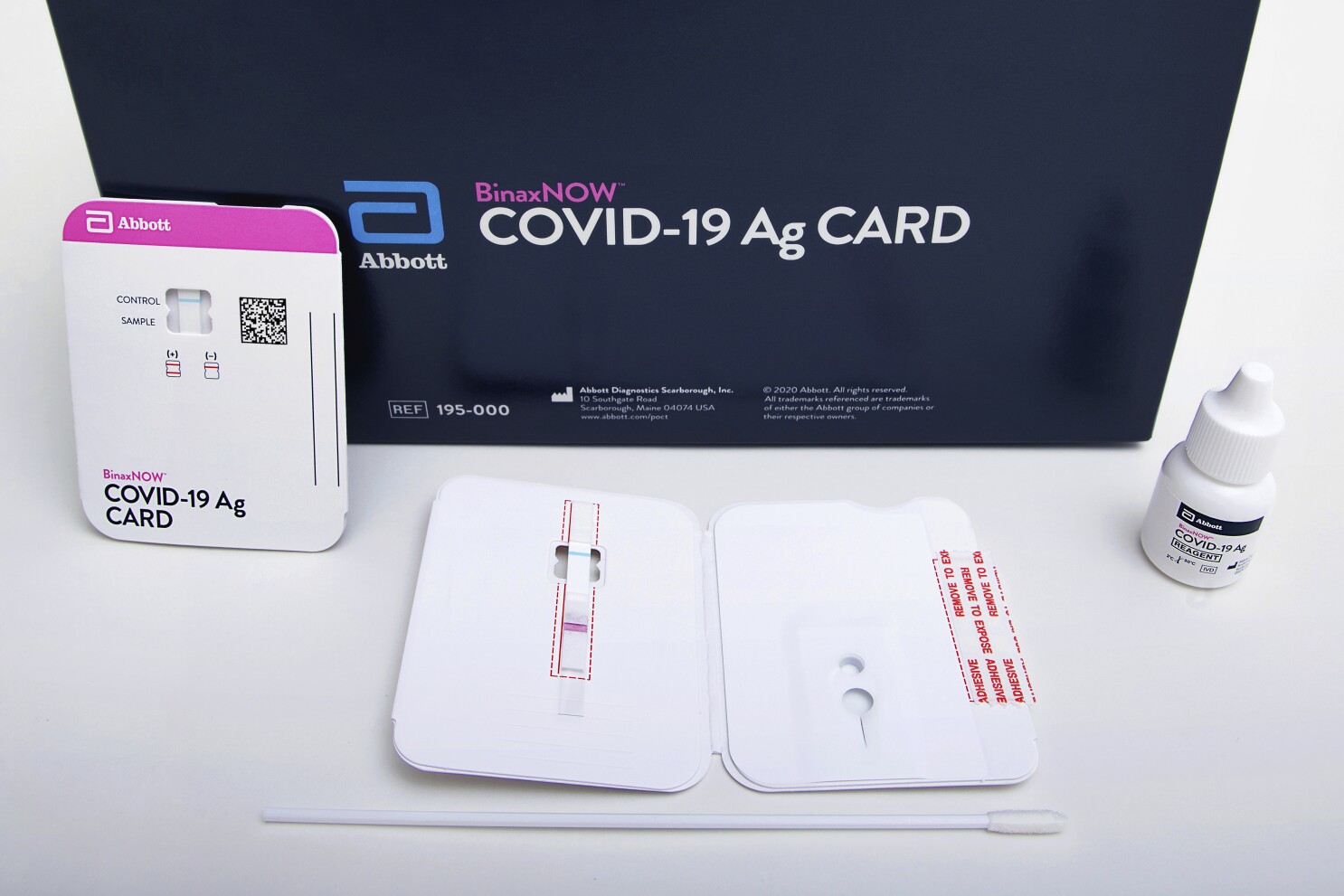
. HPB_Abbott Panbio_Antigen Rapid Test SO_A3 Poster_EN_FA NoBleed Created Date. Only the swabs provided with the kit are approved for use. Find out more about this innovative technology and its impact here. MIDASPOT COVID-19 Antibody Combo Detection Kit.
BinaxNOW COVID-19 Self-Test at Home Kit offers rapid results in the convenience of your home. Ohio has invested in rapid COVID-19 testing with a goal of using fast free and convenient testing to control the spread of COVID-19. Test results in 15 minutes. 50 out of 5 stars 2.
COVID-19 Rapid Test Kits Market Regional and Country Analysis 71. ABT announced today that the US. Store kit at 2-30C 356-86 F. 26 out of 5 stars 5.
Panbio COVID-19 Ag Rapid Test Device Nasal 25T 41FK11. Ellumes rapid COVID-19 test can send results to your smartphone in 15 minutes. ABT announced today that the US. 26 2020 PRNewswire -- Abbott NYSE.
Abbotts BinaxNOW test is contained entirely on a paper booklet. It has been available within MemorialCare for about two weeks and there remains some confusion about its proper use in testing. Abbott Panbio COVID-19 antigen rapid test SARS-CoV-2 Canada public health guidance. A simple solution for COVID-19 infection detection this 15-minute test can be completed anytime anywhere.
To find out more about how Abbotts rapid COVID-19 test works TPG spoke with Dr. 25 testsbox Buffer Solution. CDC study says Abbotts rapid COVID-19 antigen test may miss two-thirds of asymptomatic cases. By Conor Hale Jan 21 2021 325pm.
It has been authorized by the FDA under an emergency. This interview has been edited for clarity. Abbott Panbio COVID-19 Rapid Test is recommend as a Point of Care Test POCT and as such has to be performed by a healthcare professional provider or someone who has received appropriate training please refer to the Purchase Eligibility session below. This is typically a highly.
Global COVID-19 Rapid Test Kits Market Split by Region Historic and Forecast 2015-2020 2020-2025F 2030F Billion 72. Panbio COVID-19 Ag Rapid Test Device Nasal with 2D barcode 25T 41FK21. This document prepared December 12 2020 provides interim guidance on the use of the Abbott Panbio COVID-19 Antigen Rapid Test in the context of the Canadian public health system and a coordinated national response to the coronavirus disease 2019. The app then reports the results to public health experts.
Abbott will sell this test for 5It is highly portable about the size of a credit card affordable. Like Abbotts BinaxNOW test the Quidel antigen test also works like a pregnancy test. Fast identification of potentially contagious individuals. The state is working with local health departments.
All Products 991 Search Within. This Abbott BinaxNow COVID-19 Ag Card Test is perfect for larger gatherings such as those returning to workplaces conferences and professional sporting events. Abbott Panbio COVID-19 Antigen Rapid Test Device. Panbio COVID-19 Ag Rapid Test Device Nasopharyngeal 25T 41FK10.
1 offer from 149900. Abbott BinaxNOWTM COVID-19 Ag Card Test Helpful Testing Tips continued Before the Test. Rapid antigen test results are. The Abbott ID Now test utilizes a nucleic acid molecular detection method similar to their influenza A and B tests.
The test provides preliminary test results. 1700test 25-4975 tests 1-199 boxes 1600test 5000-9975 tests. If stored in a refrigerator allow time to warm up to room temperature. Extra solution is NOT available.
Place a bulk order. Mary Rodgers the principal scientist at Abbott and an infectious disease research and diagnostics through video chat. It is a rapid antigen test not a PCR test and is authorized for use in settings operating under a CLIA Certificate. The portable rapid molecular ID NOW COVID-19 test has emerged as a critical part of this arsenal allowing fast diagnosis with results in 13 minutes or less in a variety of locations such as physicians offices urgent care clinics and other point-of-care locations.
Below are details about the various partners we are working with to make testing available as well as information for employers who are interested in initiating testing programs. A second Abbott rapid test also offers quick results but cant be used for travel. Manufacturer Name McKesson Brand 17 3M 4 Abbott Rapid Dx North America LLC 255 Alfa Scientific Designs Inc 20 Arlington Scientific 42 Avanos Medical Sales LLC 3 BD 14 BD Primary Care 13 BTNX 15 Cardinal 10 Chematics 2 Chembio. ABBOTT PARK Ill Dec.
The Abbott ID Now Rapid Molecular Test for COVID-19 is the first in-house lab testing available to MemorialCare. Tests should be administered by a medical professional or trained administrator. Test kit reagentscards must be at room temperature before use. 1 bottlebox Volume Pricing.
The minimum order quantity is 25 tests 1 box. ABBOTT PARK Ill Aug. Expected Date in Our Warehouse. Do not freeze kits.
The BinaxNOW COVID-19 Ag Card 2 Home Test is a lateral flow immunoassay intended for the qualitative detection of nucleocapsid protein antigen from SARS-CoV-2 in direct anterior nasal nares swabs from individuals with or without symptoms or other epidemiological reasons to suspect COVID-19 infection when tested twice over three days with at least 36. Each box only contains one bottle of buffer solution to share between 25 tests. BinaxNOW COVID-19 Antigen Home Rapid Self-Testing Kit by Abbott 2 Tests 3895 2495-36 New. Food and Drug Administration FDA has issued Emergency Use Authorization EUA for its BinaxNOW COVID-19 Ag Card rapid test for detection of COVID-19 infection.
Panbio COVID-19 Ag Rapid Test Device is for professional use only and is intended to be used as an aid in the diagnosis of SARS-CoV-2 infection. Negative results dont preclude. 25 test kits and buffer ampules per box. Any trained person can.
If youre interested in rapid testing solutions contact us today for a quoteYou can also place an order for the Abbott Panbio COVID-19 Rapid Antigen Test Device nasal or other rapid tests approved by Health Canada through our online rapid test shop. The BinaxNOW COVID-19 Antigen Self Test has not been FDA cleared or approved. Next you place the swab in. 16 2020 PRNewswire -- Abbott NYSE.
Abbott Panbio COVID-19 Antigen Rapid Test. Our team at Rapid Test Trace Canada can also assist with implementing a. IHealth COVID-19 Antigen Rapid Test 90 Packs2 Tests per PackFDA EUA Authorized OTC at-Home Self Test Results in 15 Minutes with Non-invasive Nasal Swab Easy to Use No Discomfort. DxTerity COVID-19 Saliva at-Home.
The product may be used in any laboratory and non-laboratory environment that meets the requirements specified in the Instructions for Use and local regulation. Simply test yourself twice within 3 days with at least 36 hours between. 1 offer from 30000. Cassette Time to result.
Therefore rapid antigen tests are commonly used in workplaces as they can detect whether anyone can unknowingly spread Covid-19 to someone else. The Rapid Antigen Test is ideal for quickly determining if you are at risk of spreading the virus as it is highly sensitive in detecting viral levels that are currently considered to be infectious. Food and Drug Administration FDA has issued Emergency Use Authorization EUA for virtually guided at-home use of its BinaxNOW COVID-19 Ag Card rapid test for detection of COVID-19 infection. Quidel QuickVue rapid test.
Search rapid covid test Search Results for rapid covid test rapid covid test. Abbott conducted a computational analysis of the detection of multiple SARS-COV-2 Strains including the Delta variant and predicts no impact to the performance of our BinaxNOW COVID-19 Antigen Self Test. Patient-friendly nasal self-collected swab option. First you take a nasal swab then mix the swab with a tube of liquid for one minute.

New Rapid Tests Aimed At Limiting Covid 19 Spread During The Holidays Rochesterfirst

Fda Authorizes Covid 19 Test That Doesn T Need Special Equipment Los Angeles Times

Fda Authorizes 2 Over The Counter Coronavirus Tests Coronavirus Updates Npr

Covid 19 Rapid Test By Abbott Labs Given Emergency Fda Approval Claims Accurate Results In 15 Minutes Abc7 Chicago

Posting Komentar untuk "abbott rapid covid test"